Vitamin C Important Functions
Open access peer-reviewed chapter
Vitamin C: Sources, Functions, Sensing and Analysis
By Sudha J. Devaki and Reshma Lali Raveendran
Submitted: November 10th 2016 Reviewed: June 20th 2017 Published: August 2nd 2017
DOI: 10.5772/intechopen.70162
Abstract
Vitamin C is a water-soluble compound found in living organisms. It is an essential nutrient for various metabolism in our body and also serves as a reagent for the preparation of many materials in the pharmaceutical and food industry. In this perspective, this chapter can develop interest and curiosity among all practicing scientists and technologists by expounding the details of its sources, chemistry, multifunctional properties and applications.
Keywords
- vitamin C
- biomarker
- antioxidant
- sensors
1. Introduction
Vitamin C also known as ascorbic acid (AA) is an essential nutrient in many multicellular organisms, especially in humans. Ascorbic acid is a water-soluble vitamin and is found in variable quantities in fruits and vegetables and organ meats (e.g. liver and kidney). Deficiency of vitamin C causes scurvy, widespread connective tissue weakness and capillary fragility. Among chemists, it is used as a reagent for the preparation of fine chemicals, enzymatic reagent and nanomaterials. Consequently, the detection and quantification of ascorbic acid in food samples, products and nutraceuticals is receiving overwhelming importance among researchers, medical practitioners and also in the pharmaceutical and food industry. Figure 1 shows the schematic representation of the sources and multifunctional applications of vitamin C in the metabolism of our body.
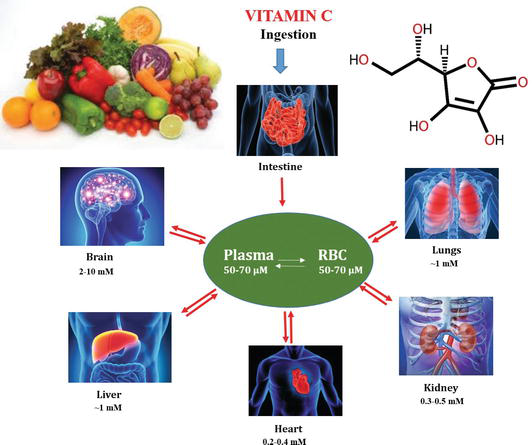
Figure 1.
Sources and multifunctional role of vitamin C in metabolic processes in human body.
Advertisement
2. Sources
Vitamin C (Figure 2) is abundantly available in many natural sources, including fresh fruits and vegetables. The richest sources of ascorbic acid including Indian gooseberry, citrus fruits such as limes, oranges and lemons, tomatoes, potatoes, papaya, green and red peppers, kiwifruit, strawberries and cantaloupes, green leafy vegetables such as broccoli, fortified cereals and its juices are also rich sources of vitamin C.

Figure 2.
Structure of vitamin C.
Another source of vitamin C is animals. They usually synthesize their own vitamin C and are highly concentrated in the liver part [1, 2]. Sources of vitamin C and its content are given in Figure 3. Average daily recommended amounts of vitamin C for different ages are also given in Table 1.
| Infants | |||||
|---|---|---|---|---|---|
| Age | Adequate intake (AI | Upper intake level (UL) | |||
| 0–6 monthsa | 25 | – | |||
| 7–12 monthsb | 30 | – | |||
| | |||||
| Age | EARc | RDId | |||
| mg/day | mg/day | mg/day | |||
| 1–3 | 25 | 35 | 400 | ||
| 4–8 | 25 | 35 | 650 | ||
| 9–14 | 28 | 40 | 1200 | ||
| 14–18 | 28 | 40 | 1800 | ||
| | |||||
| Men | Women | Both | |||
| Age | EARc | RDId | EARc | RDId | |
| mg/day | mg/day | mg/day | mg/day | mg/day | |
| 19–30 | 30 | 45 | 30 | 45 | 2000 |
| 31–50 | 30 | 45 | 30 | 45 | 2000 |
| 51–70 | 30 | 45 | 30 | 45 | 2000 |
| >70 | 30 | 45 | 30 | 45 | 2000 |
| | |||||
| Pregnancy | Lactation | Both | |||
| Age | EARc | RDId | EARc | RDId | |
| mg/day | mg/day | mg/day | mg/day | mg/day | |
| 14–18 | 38 | 55 | 58 | 80 | 2000 |
| 19–30 | 40 | 60 | 60 | 85 | 2000 |
| 31–50 | 40 | 60 | 60 | 85 | 2000 |
Table 1.
Dietary intake of vitamin C [3–5].
aCalculated as per the average intake of breast milk.
bCalculated on a body weight basis of infants.
cEstimated average requirement.
dRecommended dietary intake.
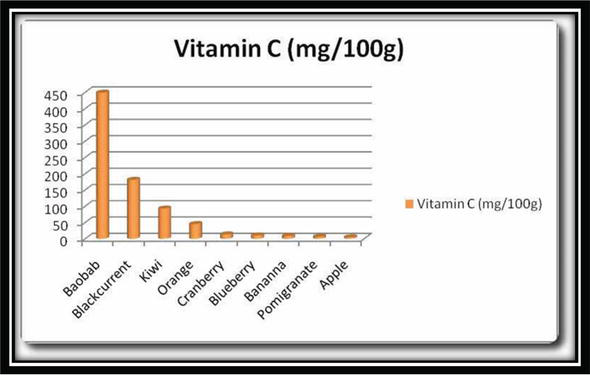
Figure 3.
Amount of vitamin C in different sources.
Advertisement
3. Functions
Vitamin C plays an important role in many physiological processes in humans. It is needed for the repair of tissues in all parts of the body. The important functions of vitamin C include the formation of protein used to make skin, tendons, ligaments, and blood vessels for healing wounds and forming scar tissue, for repairing and maintaining cartilage, bones, and teeth and aid in the absorption of iron. It can also act as a reducing and capping agent for metal nanoparticles.
3.1. As reducing and capping agent
Ascorbic acid acts as a reducing and capping agent for the synthesis of metal nanoparticles such as silver, gold, copper, etc. Ascorbic acid molecules can cap or surround the particle and prevent the uncontrolled growth of the particles to micron-sized dimensions. A study by Khan et al., in 2016 reported the synthesis of copper nanoparticle by using ascorbic acid as both the reducing agent [6]. Sun et al. reported in

Figure 4.
Analytical process for detecting dichlorvos using AA-Au NPs as a colorimetric probe [
3.2. Antioxidant activity
One of the important properties of vitamin C is its antioxidant activity. Antioxidant activity of vitamin C helps to prevent certain diseases such as cancer, cardiovascular diseases, common cold, age-related muscular degeneration and cataract.
3.3. In cancer treatment
Since 1970, it has been known that high dose of vitamin C has beneficial effects on the survival time in patients with terminal cancer, which was reported by Cameron, Campbell, and Pauling. Research is undergoing in detail for using vitamin C in cancer treatment [9–11]. One of the studies suggests that pharmacologic doses of vitamin C might show promising effects on the treatment of tumours [12]. Vitamin C can act as pro-oxidant and it can generate hydrogen peroxide [13, 14]. Administration of high dose of vitamin C gives long survival times for patients with advanced cancers.
The continuing attack of DNA by unquenched reactive oxygen species is believed to cause cancer. As a physiological antioxidant, ascorbic acid plays a role in the prevention of oxidative damage to DNA, which is elevated in cells at sites of chronic inflammation and in many pre-neoplastic lesions. Most DNA damage is repaired metabolically; however, the frequency of elevated steady-state levels of oxidized DNA bases is estimated to be sufficient to cause mutational events. The base damage product 8-hydroxy-2′-deoxyguanosine has been found to be elevated in individuals with severe vitamin C deficiency and to be reduced by supplementation with vitamins C and E [15].
Oxidative stress, arising as a result of an imbalance between free radical production and antioxidant defences, causes damage to a wide range of molecular species including lipids, proteins and nucleic acids. Oxidative stress caused by free radicals is shown in Figure 5. The best way to ensure adequate intake of the antioxidant nutrients, such as vitamins C and E and various kinds of minerals, is through a balanced diet consisting of 5–8 servings of fruits and vegetables per day.
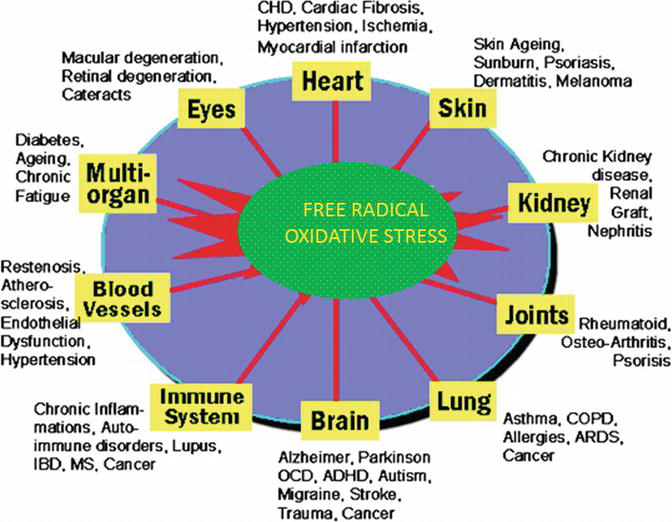
Figure 5.
Oxidative stress by free radicals.
3.4. In cardiovascular diseases
The antioxidant property of vitamin C helps for the treatment of cardiovascular diseases. Vitamin C has the capability for reducing monocyte adherence to the endothelium, improving endothelium-dependent nitric oxide production and vasodilation and reducing vascular smooth-muscle-cell apoptosis, which prevents plaque instability in atherosclerosis [15]. The oxidative damage including the oxidative modification of low-density lipoproteins is a major cause of cardiovascular disease. The antioxidant property of vitamin C helps to reduce this to a certain extent [16, 17].
3.5. In common cold
Pauling in 1970 suggested that vitamin C can be used for the treatment of common cold [18]. There are so many reports in Cochrane Database Syst. Review showing the use of prophylactic vitamin C reduces the cold duration in adults and children [19]. The use of vitamin C might reduce the duration of common cold due to its anti-histamine effect of high dose of vitamin C [20]. However, the results are inconsistent and still research is undergoing in this field. There are Database Syst. Review showing the use of prophylactic vitamin C reduces the cold duration in adults and children [19]. The use of vitamin C might reduce the duration of common cold due to its anti-histamine effect of high dose of vitamin C [20].
3.6. In age-related macular degeneration (AMD) and cataract
Age-related macular degeneration (AMD) and cataracts are two of the main causes of vision loss in older. Oxidative stress might contribute to the aetiology of both conditions. Thus, researchers have taken interest in the role of vitamin C and other antioxidants in the development and treatment of these diseases. There are many reports to study the role of vitamin C in AMD and cataract [21–23]. Results from two studies indicate that vitamin C intakes greater than 300 mg/day reduce the risk of cataract formation by 75% [16, 24, 25].
All the studies indicate that the vitamin C formulations might slow AMD progression and reduce the high risk of developing advanced AMD. AMD is shown in Figure 6.

Figure 6.
Age-related macular degeneration.
3.7. Antioxidant mechanism
Vitamins C can protect the body against the destructive effects of free radicals. Antioxidants neutralize free radicals by donating one of their own electrons, ending the electron-stealing reaction, as shown in Figure 7. The antioxidant nutrients themselves do not become free radicals by donating an electron because they are stable in either form or act as scavengers, helping to prevent cell and tissue damage that could lead to cellular damage and disease.
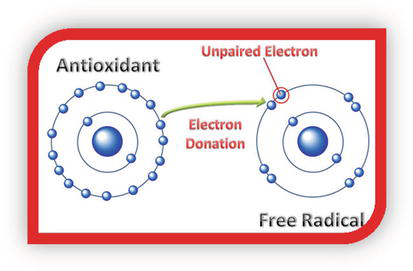
Figure 7.
Antioxidant mechanism.
Ascorbic acid reacts with free radicals undergoing single-electron oxidation to produce a relatively poor reactive intermediate, the ascorbyl radical, which disproportionates to ascorbate and dehydroascorbate. Thus, ascorbic acid can reduce toxic, reactive oxygen species superoxide anion (O2 •) and hydroxyl radical (OH•), as well as organic (RO2) and nitrogen (NO2 •) oxy radicals. Those reactions are likely to be of fundamental importance in all aerobic cells. This reaction is the basis of most of the biological functions of ascorbic acid. The mechanism of free radical action on DNA is shown in Figure 8.
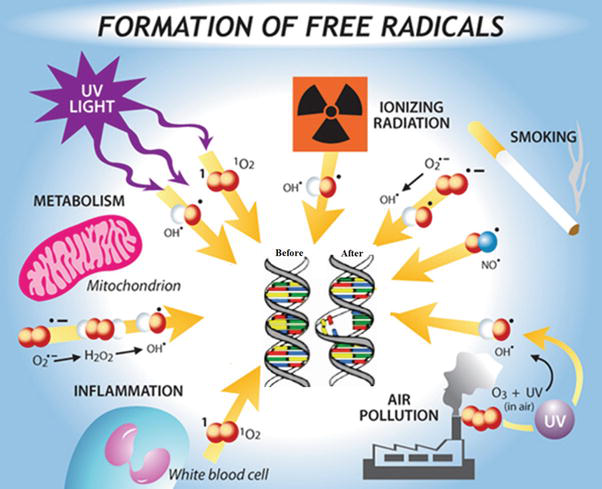
Figure 8.
Mechanism of free radical action on DNA.
Vitamin C also has role in protecting other vitamins (vitamin A and vitamin E) from the harmful effects of oxidation. Vitamin C helps in protecting gums and retards ageing. It strengthens the general physical condition by removing toxic metals from the body. Vitamin C reduces the formation of cataract and hence useful in the treatment of glaucoma.
3.8. Synthesis of protein
Another important function of vitamin C is its role in the synthesis of protein. Vitamin C helps in the synthesis of collagen. Collagen protects our skin from wrinkling and makes our skin firm and strong. Collagen also protects and supports organs and other soft tissues. One of the amino acids used to build collagen—hydroxyproline—is only synthesized when vitamin C is available. Functions of vitamin C on skin.
3.9. Functions of vitamin C on skin
3.9.1. Photoprotection
Vitamin C reduces the damage caused by UV-light exposure. It cannot act as a sunscreen since it cannot absorb UV light. But the antioxidant activity of vitamin C helps to protect UV damage caused by free radicals [26]. In response to UV light, vitamin C transport proteins are increased, suggesting an increased need for vitamin C uptake for adequate protection [27, 28]. Addition of keratinocytes on vitamin C reduces damages caused by UV light and lipid peroxidation, limits the release of pro-inflammatory cytokines and protects against apoptosis [29]. Many studies have suggested that vitamin C consumption alone will not reduce the effect of UV exposure; however, a combination of vitamin C and E effectively increases minimal erythemal dose (MED) (the lowest dose of ultraviolet radiation (UVR) that will produce a detectable erythema 24 hours after UVR exposure) and decreases erythema-induced blood flow to damaged areas of skin [30]. Thus, interactions between the two antioxidant vitamins may be necessary to achieve UV protection. The topical application of vitamin C also reduces the effect of UV exposure and skin wrinkling and skin tumour [31]. Vitamin C reduced the number of sunburned cells, decreased erythema response and reduced DNA damage induced by UV exposure [27]. The combination of antioxidant vitamins decreased the immunosuppressive effects of UV exposure, increased MED and decreased cell damage [32, 33].
3.9.2. Wound healing
Vitamin C plays a key role in healing wound by the formation of collagen, connective tissue [34–36]. The new tissue is rebuilt with the help of collagen framework. This function is supported by its co-factor vitamin C. Besides this, vitamin C performs as a strong antioxidant and immune system modulator [37, 38].
3.10. Deficiency of vitamin C
Deficiency of vitamin C in humans causes a major disease called scurvy. The major signs of the disease occur primarily in mesenchymal tissues. It leads to impaired wound healing; oedema; haemorrhage (due to deficient formation of intercellular substance) in the skin, mucous membranes, internal organs, and muscles; and weakening of collagenous structures in bone, cartilage, teeth and connective tissues. Those who suffer from scurvy have swollen, bleeding gums with tooth loss. They also show lethargy, fatigue, rheumatic pains in the legs, muscular atrophy and skin lesions, massive sheet hematomas in the thighs, and ecchymoses and haemorrhages in many organs, including the intestines, sub-periosteal tissues and eyes. All these features are accompanied by psychological changes: hysteria, hypochondria and depression. In children, the syndrome is called Moeller-Barlow disease; it is seen in non-breastfed infants usually at about 6 months of age and is characterized by widening of bone-cartilage boundaries, stressed epiphyseal cartilage of the extremities, severe joint pain and frequently, anaemia and fever. Children having this disease present with a limp or inability to walk, tenderness of the lower limbs, bleeding of the gums and petechial haemorrhages.
Advertisement
4. Detection and sensing
Many analytical techniques are used for the determination of vitamin C in different matrices, such as titrimetric [39], fluorimetric [40], spectrophotometric [41], high-performance liquid chromatography [42], enzymatic [43], kinetic [44, 45] and electrochemical, etc.
4.1. High-performance liquid chromatography (HPLC)
High-performance liquid chromatography (HPLC) methods are preferred earlier because they are faster and more effective than spectrophotometric, titration or enzymatic methods, and they do not usually need derivatization [46]. In pharmaceutical and cosmetic industries, HPLC is used, which is considered as a sensitive and selective method.
Detection of vitamin C through HPLC has been done by many groups. Racz et al. in 1990 reported that HPLC used the HPLC method for the determination of ascorbic acid in fruits and vegetables. The method has been used to deep-frozen raspberry cream analysis together with three commonly used chemical methods of vitamin C analysis. The HPLC method has been compared with the chemical methods from several aspects, and the superiority of HPLC method has been concluded [47].
Snezana et al. reported the HPLC method for the determination of vitamin C in pharmaceutical samples in

Figure 9.
HPLC plot of ascorbic acid detection.
Another work carried out by Franco et al., which was to optimize and validate a new analytic strategy for the determination of vitamin C in strawberries by UV–HPLC [49].
For the accurate and reliable measurement of ascorbic acid and dehydroascorbic acid, HPLC can be used in combination with electrochemical or ultraviolet detection. Line and Hoffer have reported a method for the determination of vitamin C in plasma. It can be analysed by HPLC in connection with electrochemical or ultraviolet light detection. Electrochemical HPLC has advantages over UV-HPLC for plasma total vitamin C analysis [50].
4.2. Spectrophotometric method
Among many analytical methods, spectrophotometric methods are very simple and low-cost. Several studies used the spectrophotometric method for the determination of ascorbic acid. Güçlü et al. [51] have proposed a spectrophotometric method based on ascorbic acid oxidation to dehydroascorbic acid, by using the Cu(II)-neocuproine complex, which is reduced to Cu(I)-bis(neocuproine), the absorbance of the latter being determined at 450 nm. Other optical methods for vitamin C estimation include spectrophotometrical determination of iodine reacted with ascorbic acid [52] and chemiluminescence [53].
A sensitive, simple and low-cost spectrophotometric method was introduced by Kobra and Somayye in 2015. The present method was successfully applied to determine the ascorbic acid in food and pharmaceutical samples. The samples were multivitamin tablet, effervescent tablet, vitamin C injection, natural orange juice, orange syrup powdered and commercial orange liquid. The method is based on the reaction of AgNO3 with ascorbic acid in the presence of polyvinyl pyrrolidone (PVP) and slightly basic medium to prepare silver nanoparticles [54].
Kapur et al. in 2015 reported a method that is based on the oxidation of ascorbic acid to dehydroascorbic acid by bromine water in the presence of acetic acid. In this method, the total ascorbic acid (ascorbic acid + dehydroascorbic acid) has been determined in 21 different samples of fruits and vegetables by the spectrophotometric method [55]. Mohammed and Hazim in 2016 reported a UV-spectrophotometric method for the determination of ascorbic acid in fruits and vegetables from hill region with 2,4-dinitrophenylhydrazine [56].
A new sensitive colorimetric method for the determination of ascorbic acid tablet in aqueous solution was reported by Ahmed and Mohamed in 2013. The method is based on the formation of coloured azo dye by diazotization of 2,4-dichloroaniline, followed by azo-coupling reaction between the resulting product and ascorbic acid [57].
4.3. Electrochemical sensing
Electrochemical sensing methods are more widely applied as they are simple, sensitive and moderate methods. Ascorbic acid (AA), dopamine and catecholamine are important neurotransmitters in the human body. Hence, the low-level detection of ascorbic acid is very important. All the methods mentioned above involve complicated pretreatment techniques and expensive instruments. Hence, researchers have developed a more simple, sensitive and accurate electrochemical method for the detection of ascorbic acid. Several studies have reported the electrochemical sensing methods for detection of ascorbic acid. Attempts to simplify such methods have resulted in the development of new methods such as the nickel hexacyanoferrate film-modified aluminium electrode [58] and graphite epoxy electrode [59]. These methods use specific and modified working electrode systems that are complicated [60]. These electrochemical methods are widely used to monitor AA metabolites
Suw et al. in 2004 developed a stripping voltammetric technique that manifests faster response, more cost-effective and sensitive preconcentration techniques, which was used along with a simpler glassy carbon electrode [68]. In this method, low ascorbic acid concentrations were detected by the square wave stripping voltammetry and a glassy carbon electrode. The lower detection limit reported as 0.30l g/L (S/N = 3). This method can be used to detect biological materials, pharmaceuticals, food and drugs.
Many reports are there for the simultaneous electrochemical detection of ascorbic acid in the presence of dopamine and uric acid on the glassy carbon electrode. From our own group, we have synthesized electrically conducting poly (3,4-ethylenedioxythiophene) nanospindles (PEDOTSs) and used for the electrochemical sensing of ascorbic acid [69] (Figure 10).

Figure 10.
Cyclic voltammogram of electrochemical sensing of ascorbic acid using a polyaniline-modified platinum electrode [
In another study, our group developed a low-cost electrochemical sensor based on a platinum electrode for ascorbic acid conductive polyaniline-based composite. This unique low-cost and user-friendly sensor was validated for the nanomolar detection of AA. The lower detection limit for AA was observed at 0.1 nm. The aqueous PPICS/Pt electrode could serve as a prospective low-cost and efficient electrochemical sensor and provide promising and outstanding contributions to the food, beverage and medical industries [70].
Electrochemical methods have attracted much attention from clinical diagnostic perspectives because of their easy operation, low cost, rapid response, high sensitivity and good selectivity.
Advertisement
5. Conclusion
Vitamin C plays a pivotal role in body-building process and in disease prevention. The various functions of vitamin C, including the antioxidant activity, formation of protein, tendons, ligaments and blood vessels, for healing wounds and form scar tissue, for repairing and maintaining cartilage, bone, and teeth, and aiding in the absorption of iron, were discussed. This chapter will definitely benefit the students, researchers and technologists globally.
© 2017 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
How to cite and reference
chapter statistics
5815 total chapter downloads
9 Crossref citations
More statistics for editors and authors
Login to your personal dashboard for more detailed statistics on your publications.
Access personal reporting
Related Content
This Book
Next chapter
Vitamin C: An Antioxidant Agent
By Fadime Eryılmaz Pehlivan
Related Book
First chapter
Role of an Atomic-Level-Based Approach for Improving Cancer Therapy
By Santi Tofani
We are IntechOpen, the world's leading publisher of Open Access books. Built by scientists, for scientists. Our readership spans scientists, professors, researchers, librarians, and students, as well as business professionals. We share our knowledge and peer-reveiwed research papers with libraries, scientific and engineering societies, and also work with corporate R&D departments and government entities.
More About Us
Source: https://www.intechopen.com/chapters/56440







Tidak ada komentar:
Posting Komentar